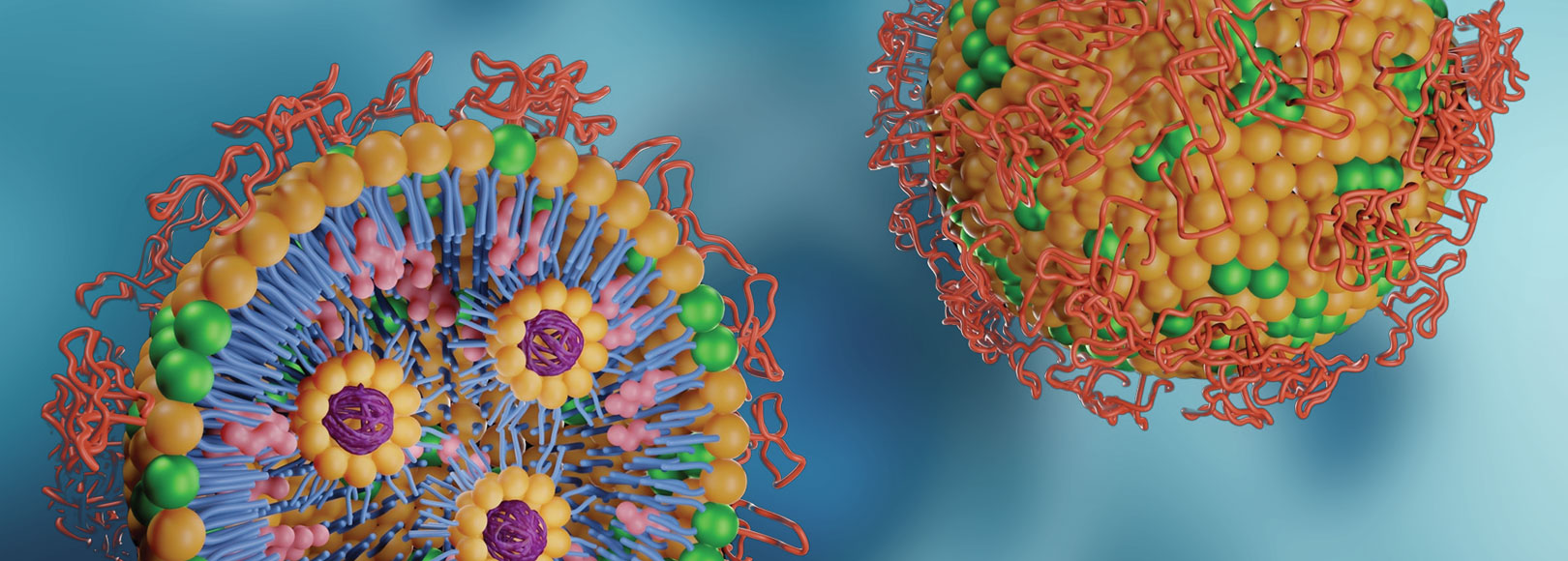
Contract Development Organization
Raw Material Research and Development Service for customers who want to differentiate themselves from raw materials.
Dx-Cloud™
Service providing strain genome database accumulated through multi-omics data analysis.
Microbial analysis

Genome analysis

Metabolite analysis

Dx-Cloud™
- Provides a microbial target genome database service.
- Provides Dx-Cloud™ data service for customers planning new projects.
Vxcovery™
Research service for studying the functionality of separated microorganisms and confirming their characteristics through Dx-Cloud™.

100 strains from dairy products

300 strains from fermented foods
600 strains from human bodies

1,000 strain library

Research on health functionality effects

Research on functional action mechanisms

Establishment of commercialization conditions
- Know-how accumulated by studying and isolating about 1,000 microorganisms from various samples
- Customized strain research and development services based on the concept of customers who want to develop new probiotic ingredients
Process Optimization
Process development services for all strains with over 900 pieces of process optimization know-how.

Case Study on Process Optimization
Suwon Lee
Director, Microbiome R&D ceter
- D. Zhang., S. Lee, C. Lim. et al. Isolation and characterization of new probiotic strains from Chinese babies. J Clin Gastroenterol. 52(1):S27-S341.
- S. Zhang, S. Lee, C. Lim et al. Probiotic characterization of Lactobacillus plantarum HOM3204 and its restoration effect on antibiotic-induced dysbiosis in mice. Lett Appl Microbiol. 74: 949-958. 외 200편의 논문
Development of Strains and Production Processes for Mamiai (妈咪爱®) Products by Beijing Hanmi Pharmaceutical
- Development of Strain Cultivation Processes
- Development of Spore Formation Conditions
- Development of Freeze-drying Protectant Conditions
- Registration with the China Food and Drug Administration (CFDA)
- Case Study of the Development of Strain Production Processes and Registration with the CFDA for Mamiai (妈咪爱®) Products by Beijing Hanmi Pharmaceutical
- Construction of a Database comprising 900 Cultivation Conditions/Freeze-drying Protectant Compositions/Spore Formation Conditions
- Development of Production Processes for Customers who possess Functional Strains but lack Production Processes
Formulation Development
Finished product development service that takes responsibility for selecting customized ingredients and conducting regulatory reviews for product type/form.
Product Types

Pharmaceuticals

Health Functional Foods

General Foods

Cosmetics
Finished Product Development

Product Forms

Capsule Form

Powder Form

Liquid Form
Granule Form
- Selection of ingredients and mixing ratios that meet the specifications for each product type/form
- Development of product forms that fit the distribution lines of pharmaceutical/health functional food/cosmetic sales customers
- Regulatory review service that takes responsibility for final product completion and registration